Start of topic | Skip to actions
Numerical Simulations of Gaseous Detonations
Detonations with One-Step Chemistry
A popular model is a reaction mechanism just of one exothermic reaction
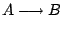
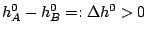 and the reaction rate
and the reaction rate
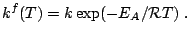
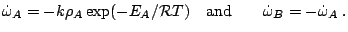
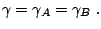
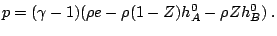
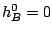
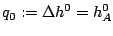
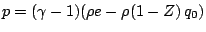
Subsections
-- RalfDeiterding - 14 Dec 2004
Copyright © 1997-2025 California Institute of Technology.